Which Element or Compound Produced a White-gray Flame
Which element or compound produced a white-gray flame quizlet why do different elements produce different colors of light when heated did potassium nitrate and potassium dihydrogen arsenate produce similar colored flames. A flame test is the simplest way of identifying the presence of group 1 metal ions in the compound.
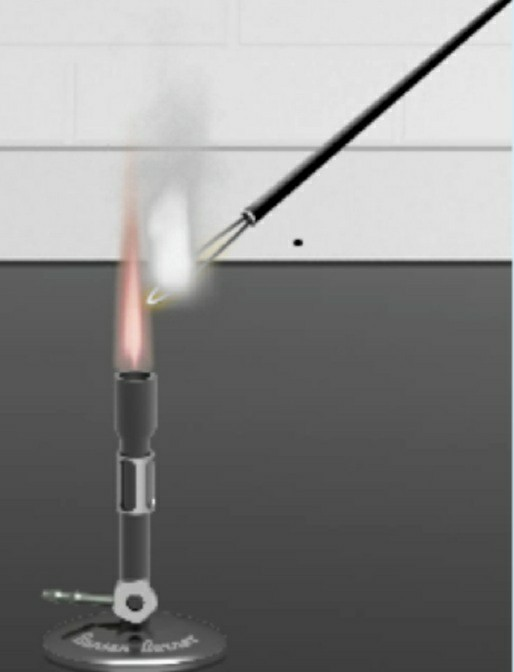
Magnesium is bright and white.

. As Se In Cu. You can buy Crystal Color Fire Sticks for 499 from Amazon that produce bright greens blues and reds in a fireplace or campfire. If you melt sulfur you get a blood-red liquid.
A flame test is an analytical procedure used in chemistry to detect the presence of certain elements primarily metal ions based on each elements characteristic emission spectrum. View Lab Report - ch 6 lab flame testdocx from CHEMISRTY 111 at Pentecostal Life University. What color flame did lead nitrate produce.
Which element or compound produced a white flame. A platinum or nichrome wire is used formed into a loop to hold the substance being tested and then cleaned using dilutes hydrochloric acid. See flame color.
For example copper will yield a blue flame iron will give us a gold color and magnesium will yield a brilliant white. AmagnesiumBarsenic acidCpotassiumDbismuth For which. Which compound produced a purple flame.
The solid is bright yellow. Question 6 What color flame did zinc produce. Who are the experts.
A good example of a white flame is the flame with which a magnesium wire burns. When you get right down to it most the chemical elements have a ho-hum appearance. We review their content and use your feedback to keep the quality high.
When magnesium wire is burnt in airoxygen it burns with a bright white. Salts are used among other things to provide color in the crafting of pyrotechnics. A flame test showing the presence of Lithium.
A mixture of strontium red compounds and copper blue compounds produces purple. Helpful 0Not Helpful 0. This problem has been solved.
Given that the molar mass of the anhydrous calcium sulfate is 13614 gmol the molar mass of the hemihydrate is 14515 gmol and the molar mass of water is 18015 gmol what is the. What is seen by the eye is not the color absorbed but the complementary color from the removal of the absorbed wavelengthsThis spectral perspective was first noted in atomic spectroscopy. Which compound produced a purple flame.
See answer 1 Best Answer. See the answer See the answer done loading. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in the sample.
Salts of Iron Salts of Aluminum etc. In industry the flame test is very useful for the identification of polymers. You can test sodium carbonate and calcium carbonate to prove to yourself that it is the metallic part the cation of the compound producing the color.
Experts are tested by Chegg as specialists in their subject area. What element is most likely present in the sample tested below. The noble metals gold silver platinum palladium and some other elements do not produce a characteristic flame test color.
Which element or compound produced a white flame. Silverwhite can be produced by. Which compound produced a purple flame.
Arsenic selenium indium copper halides ok. They are more useful for some metals than others. Yes it is possible to have a white flame.
For which compound was the flame produced not a shade of green. Copper compounds produce blue. Which element or compound produced a white flame.
Particularly for the Group 1 metals they provide a good way of quickly identifying the metal ion present. There are several possible explanations for this one being that the thermal energy isnt sufficient to excite the electrons of these elements enough to release energy in the visible range. Question 6 what color flame did zinc produce select.
The color of flames in general also depends on temperature and oxygen fed. Magnesium potassium and titanium all elemental metals are commonly used in fireworks to produce bright white lights. Which element or compound produced a white-gray flame.
If you want to make a fire change colour then the best way is to put metallic salts on the fire. Calcium sulfate is a white solid found as two hydrates a hemihydrate known as plaster of Paris and a dehydrate known as gypsum. Flame Test Question 1 Which element or compound produced a white-gray flame.
Flame tests are utilised in chemistry to identify the metal ions in compounds. Metal salts are chemical compounds produced in an Acid Bath by immersing a metal in a mixture of acid and potash which is an alkali. Not all metal ions emit colour when heated in the gas burner.
For other metals there are plenty of reliable techniques but a flame. For which compound was the flame produced not a shade of green. Every type of metal produces its own salt eg.
If you set it on fire you get a blue flame. The color of chemicals is a physical property of chemicals that in most cases comes from the excitation of electrons due to an absorption of energy performed by the chemical. Which element or compound produced a white-gray flame.
The hemihydrate is a white solid as shown in the figure below. Aluminum magnesium titanium or antimony III. Previous question Next question.
Flame Test Question 7 Which compound produced a light blue flame.

Element Compound Or Mixture 1 2 1 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams

Comments
Post a Comment